Brain Tumors
LATEST CLASSIFICATION FOR BRAIN TUMORS (ADULTS AND CHILDREN)
DR PREM PILLAY, SENIOR NEUROSURGEON AND BRAIN TUMOR EXPERT
(HIGHER TRANING AND SUPER SPECIALIZATION IN ADULT AND PEDIATRIC BRAIN TUMORS AT THE CLEVELAND CLINIC, MD ANDERSON CANCER HOSPITAL, USA AND THE HOSPITAL FOR SICK CHILDREN, TORONTO)
The 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS), also known as WHO CNS5, represents a significant update in the classification and diagnosis of brain tumors. This fifth edition builds upon the 2016 classification, incorporating new molecular insights and diagnostic approaches. Here is a comprehensive summary of the key changes and features of the latest WHO brain tumor classification:
General Changes and Principles
Integrated Diagnosis
The WHO CNS5 emphasizes the importance of integrated diagnoses, combining histological, molecular, and clinical information. This approach aims to provide more accurate and clinically relevant tumor classifications.
Layered Reporting
The classification introduces a layered reporting structure, including:
- Integrated diagnosis
- Histological classification
- CNS WHO grade
- Molecular information
This structure allows for a more comprehensive and nuanced understanding of each tumor which allows a better selection of treatment options for patients.
Grading System
The classification now uses Arabic numerals (1, 2, 3, 4) instead of Roman numerals for tumor grades. Importantly, grading is now done within tumor types rather than across different types. This change aims to better reflect the biological behavior of specific tumor entities.
Molecular Diagnostics
WHO CNS5 significantly expands the role of molecular diagnostics in tumor classification. Many tumor types now require molecular testing for accurate diagnosis and grading. This is important for a more personalized approach to the treatment of these tumors with potentially better outcomes.
Major Changes in Tumor Classification
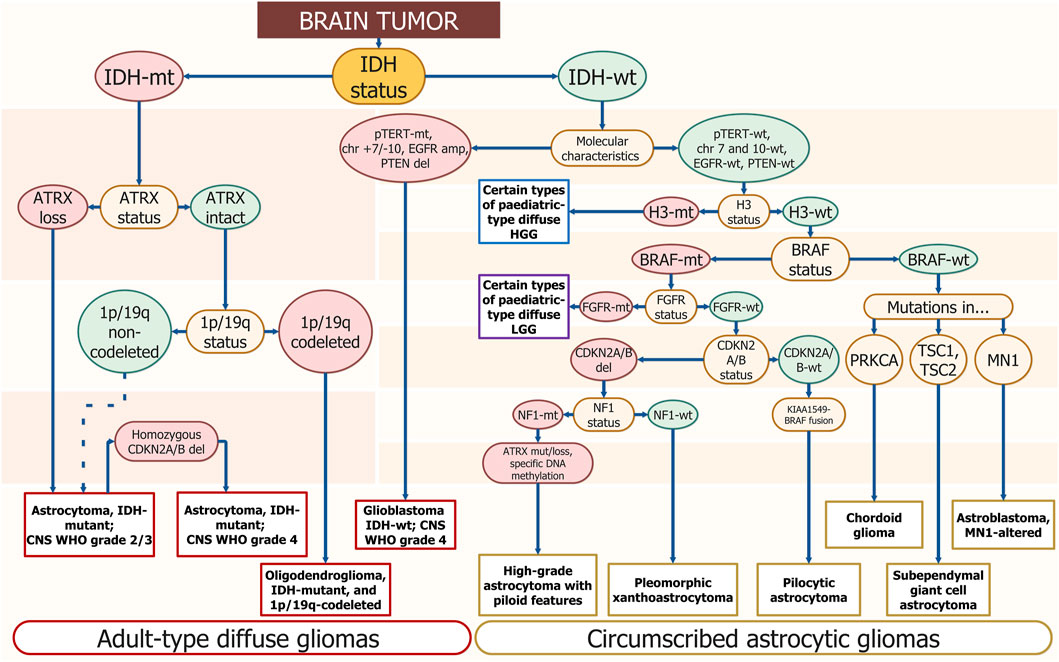
Adult-Type Diffuse Gliomas
- Astrocytoma, IDH-mutant
-
- Grades: 2, 3, or 4
- Key molecular markers: IDH1/2 mutation, ATRX loss, TP53 mutation
- CDKN2A/B homozygous deletion indicates grade 4.
- Oligodendroglioma, IDH-mutant and 1p/19q-codeleted
- Grades: 2 or 3
- Key molecular markers: IDH mutation, 1p/19q codeletion, TERT promoter mutation.
- Glioblastoma, IDH-wildtype
- Always grade 4
- Molecular markers for diagnosis: EGFR amplification, TERT promoter mutation, or combined gain of chromosome 7 and loss of chromosome 10.
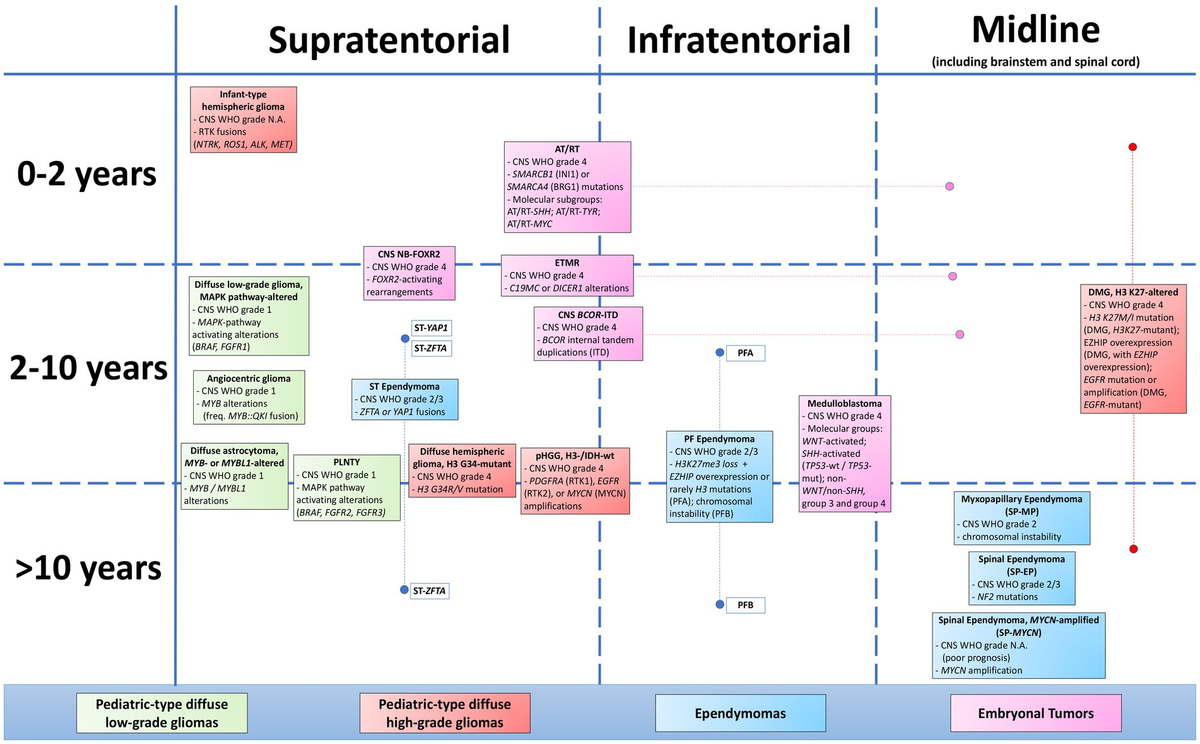
Pediatric-Type Diffuse Gliomas
- Diffuse astrocytoma, MYB- or MYBL1-altered
- Angiocentric glioma
- Polymorphous low-grade neuroepithelial tumor of the young
- Diffuse low-grade glioma, MAPK pathway-altered.
Pediatric-Type High-Grade Gliomas
- Diffuse midline glioma, H3 K27-altered
- Diffuse hemispheric glioma, H3 G34-mutant
- Infant-type hemispheric glioma.
Circumscribed Astrocytic Gliomas
- Pilocytic astrocytoma
- High-grade astrocytoma with piloid features
- Pleomorphic xanthoastrocytoma
- Subependymal giant cell astrocytoma.
Glioneuronal and Neuronal Tumors
This category includes various entities such as ganglioglioma, dysembryoplastic neuroepithelial tumor, and rosette-forming glioneuronal tumor, among others.
Ependymal Tumors
The classification of ependymal tumors now incorporates molecular subgroups, particularly for posterior fossa ependymomas.
Choroid Plexus Tumors
These tumors are now graded within their tumor type, with molecular features playing a role in diagnosis and prognosis.
Embryonal Tumors
- Medulloblastoma: Now classified into molecularly defined subgroups (WNT-activated, SHH-activated, and non-WNT/non-SHH).
- Atypical teratoid/rhabdoid tumor
- Embryonal tumor with multilayered rosettes
- CNS neuroblastoma, FOXR2-activated
Molecular Diagnostic Tools
The WHO CNS5 classification relies heavily on molecular diagnostic tools for accurate tumor classification. Some key methods include:
- DNA and RNA Next-Generation Sequencing: For detecting mutations, fusions, and copy number alterations
- Methylome Profiling: Particularly useful for tumor classification and identification of novel entities.
- Immunohistochemistry: For detecting protein expression of key molecular markers (e.g., IDH1 R132H, ATRX, H3 K27M)
- Fluorescence In Situ Hybridization (FISH): For detecting chromosomal alterations like 1p/19q codeletion
Implications for Clinical Practice
- Multidisciplinary Approach: The integrated diagnosis approach necessitates close collaboration between pathologists, molecular biologists, and Neurosurgeons.
- Personalized Medicine: More precise tumor classification allows for more targeted therapies and better prognostication and potentially better outcomes.
- Challenges in Implementation: The increased reliance on molecular testing may pose challenges in resource-limited settings.
- Ongoing Research: The classification acknowledges that our understanding of CNS tumors is evolving, and future updates are anticipated as new molecular insights emerge.
In conclusion, the WHO CNS5 classification represents a significant step towards a more molecularly informed and clinically relevant approach to brain tumor diagnosis. It integrates histological and molecular features to provide a comprehensive tumor classification system that aims to improve patient care through more accurate diagnosis, prognosis, and treatment selection.